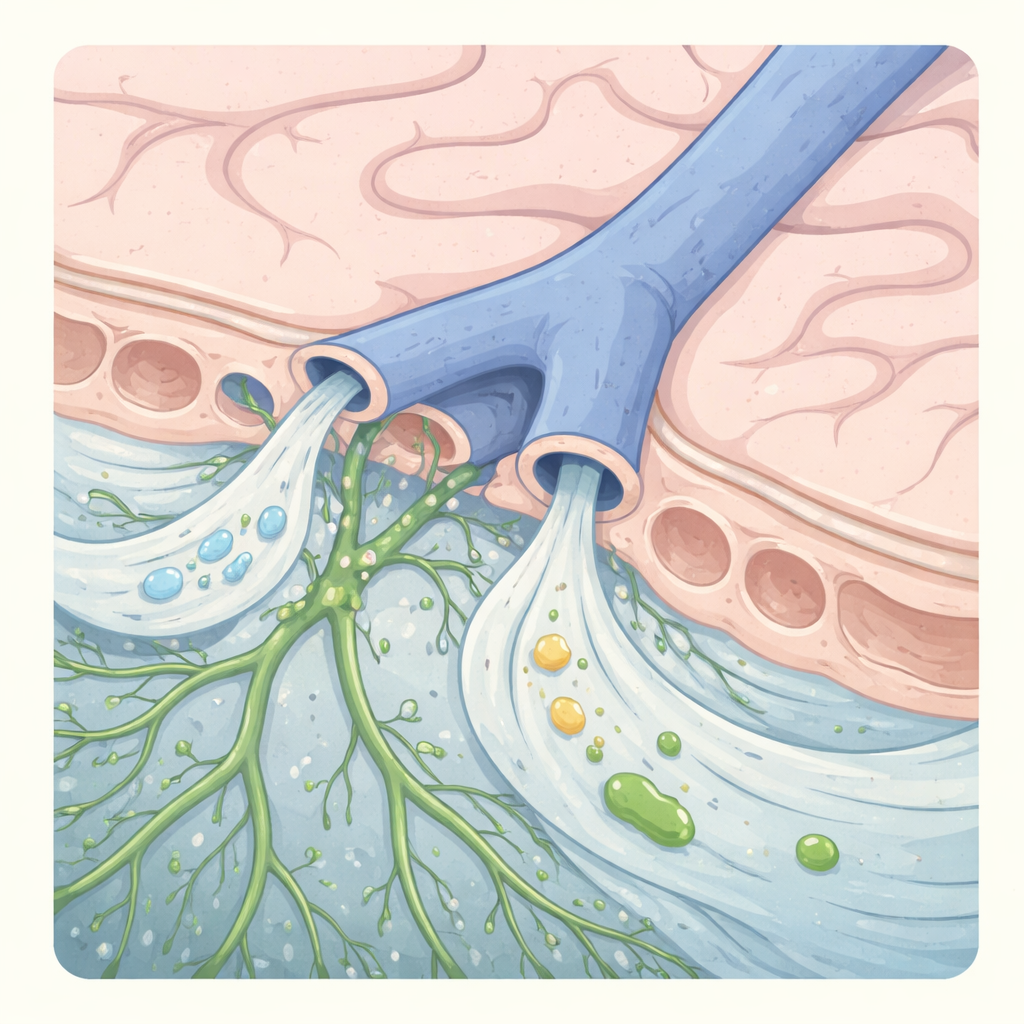
The classical view of the arachnoid barrier as a continuous, impermeable interface separating the central nervous system (CNS) from the dura mater has been increasingly challenged by discoveries in meningeal lymphatics, glymphatic flow, and neuroimmune surveillance. In a landmark 2024 study published in Nature, Smyth et al. identify discrete anatomical discontinuities in the arachnoid barrier surrounding bridging veins, termed arachnoid cuff exit (ACE) points. These structures permit bidirectional exchange of fluids, molecules, and - under inflammatory conditions - immune cells between the dura mater and the subarachnoid space. This work reframes the arachnoid barrier as a regionally specialized, dynamically regulated interface and establishes ACE points as a critical component of CNS waste clearance and neuroimmune communication in both mice and humans.
Rethinking the Arachnoid Barrier
Historically, CNS immune privilege was attributed to strict anatomical barriers, particularly the blood-brain barrier and the blood-CSF barrier. While meningeal lymphatic vessels and perineural drainage routes have since demonstrated active CNS-peripheral communication, the mechanism by which cerebrospinal fluid (CSF) and immune signals traverse the arachnoid barrier remained unresolved.
Using single-nucleus RNA sequencing, electron microscopy, tracer kinetics, and intravital imaging, Smyth et al. demonstrate that arachnoid barrier cells (ABCs) form a highly junctionalized epithelial-like layer with minimal transcytotic capacity. These findings argue against diffuse paracellular or transcellular leakage as the primary route of CSF efflux. Instead, the authors identify focal anatomical discontinuities at sites where bridging veins perforate the arachnoid barrier.
ACE Points: Structure and Function
At these vein-barrier interfaces, ABCs form a non-sealed cuff around the vessel rather than a continuous barrier. These ACE points create a permissive corridor linking the subarachnoid space directly to the dura mater. Several key functional properties emerge:
-
Bulk CSF Efflux
CSF tracers injected intracisternally appear in the dura mater before reaching blood or cervical lymph nodes, with preferential accumulation along bridging veins. Tracers spanning a wide range of molecular sizes enter the dura at similar rates, consistent with bulk flow rather than selective transport. -
Integration with Meningeal Lymphatics
ACE points are spatially coupled to dural lymphatic vessels, positioning them as upstream gateways through which CSF enters the lymphatic drainage system. This refines current models of glymphatic clearance by extending perivenous efflux beyond the brain parenchyma. -
Bidirectional Molecular Exchange
Although hydrostatic gradients favor CSF outflow, limited entry of dural or intravenously delivered molecules into the subarachnoid space occurs at ACE points. This provides a plausible anatomical basis for prior observations that dural cytokines and transcranially applied agents can influence brain physiology.
Neuroimmune Trafficking in Disease
Under homeostatic conditions, immune cell movement across ACE points is tightly restrained by stromal-derived chemorepellents such as semaphorin-3A. During neuroinflammatory states, including experimental autoimmune encephalomyelitis, this restraint is relaxed. The authors demonstrate:
-
Accumulation of neutrophils and monocytes around ACE points.
-
Directional migration of myeloid cells from the dura toward the subarachnoid space.
-
Dependence of this trafficking on extracellular matrix-integrin interactions, particularly laminin-Itga6 signaling.
Therapeutic disruption of this pathway attenuates disease severity, implicating ACE points as actionable targets in inflammatory CNS disorders.
Human Relevance
High-resolution MRI in healthy volunteers reveals tracer enhancement along parasagittal bridging veins within the subarachnoid space, mirroring findings in mice. These observations strongly suggest that ACE-like structures exist in humans and may contribute to age-related changes in CSF clearance, neuroinflammation, and susceptibility to proteinopathies.
Conceptual and Clinical Implications
The identification of ACE points fundamentally alters our understanding of meningeal anatomy and CNS barrier function. Rather than a uniform wall, the arachnoid barrier is a regionally specialized interface balancing separation and communication. ACE points likely play roles in:
-
CNS waste and macromolecular clearance
-
Immune surveillance and inflammatory cell entry
-
Leptomeningeal involvement in multiple sclerosis, meningitis, and metastatic disease
-
Age-related impairment of glymphatic and lymphatic drainage
From a translational perspective, ACE points offer a mechanistic framework for interpreting CSF-dura interactions observed in imaging and biomarker studies and may represent novel targets for modulating neuroimmune access without disrupting the blood-brain barrier.
Conclusion
Smyth et al. provide compelling anatomical and functional evidence for arachnoid cuff exit points as direct conduits between the dura mater and the brain. This discovery integrates CNS fluid dynamics, meningeal lymphatics, and neuroimmunology into a unified model and opens new avenues for understanding - and potentially treating - neurological disease.