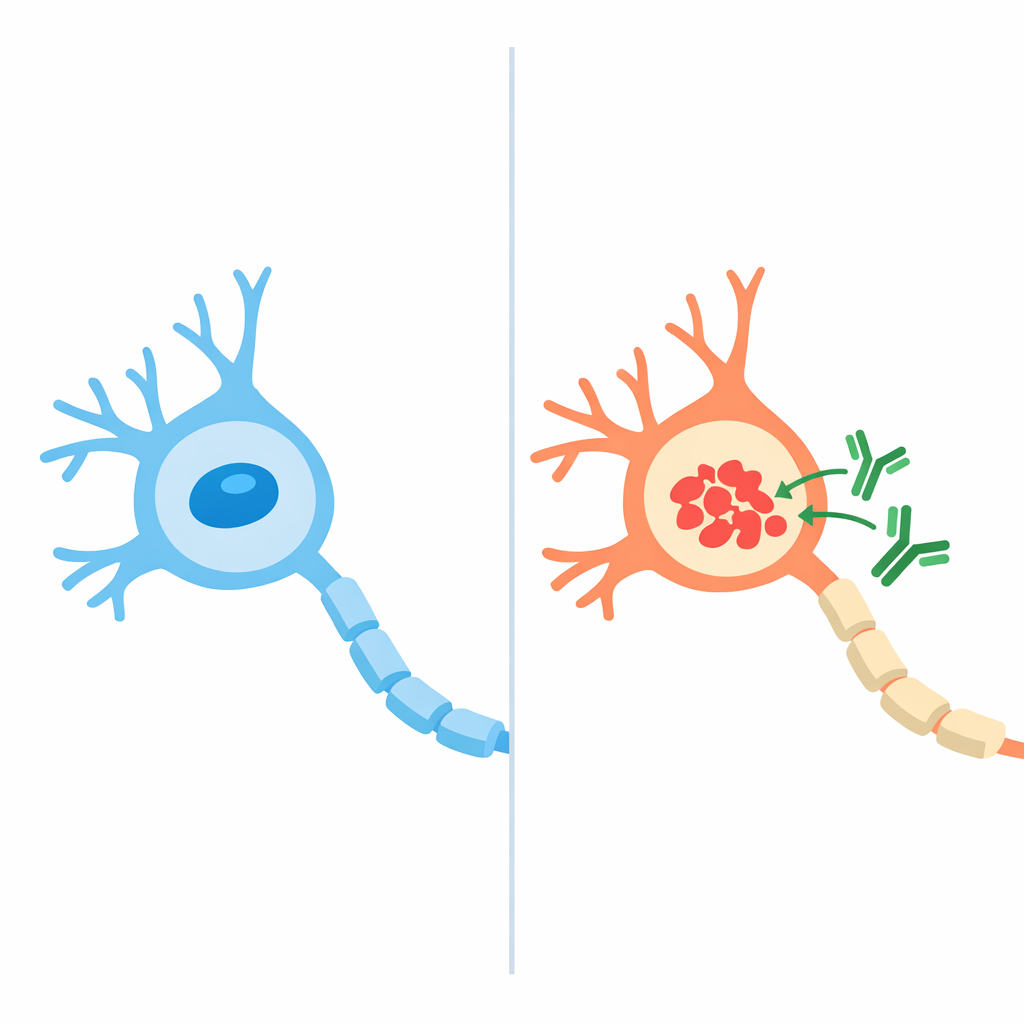
VTx-002 is a groundbreaking therapy developed by VectorY Therapeutics for treating amyotrophic lateral sclerosis (ALS). This adeno-associated virus (AAV)-delivered vectorized antibody aims to counteract the pathologic TDP-43 protein species, a critical factor in approximately 97% of ALS cases. By ensuring sustained intracellular antibody production within the central nervous system (CNS), VTx-002 overcomes the barriers posed by the blood-brain barrier and addresses both the gain of toxic cytoplasmic aggregation and the loss of essential nuclear TDP-43 function.
After extensive preclinical testing, VTx-002 has entered human trials through the PIONEER-ALS Phase 1/2 trial and has been granted Fast Track designation by the U.S. Food and Drug Administration as of early 2026. This article explores the biological basis, platform engineering, clinical development strategy, and potential applications of VTx-002, including its relevance to TDP-43-driven cognitive disorders.
1. The Central Role of TDP-43 in ALS Pathobiology
Transactive response DNA-binding protein 43 (TDP-43) is a nuclear protein essential for RNA splicing, transcriptional regulation, RNA transport, and DNA damage response. Normally, TDP-43 is found in the nucleus, maintaining RNA integrity and neuronal stability.
In ALS, TDP-43 becomes pathologically misfolded and mislocalized to the cytoplasm, forming insoluble, phosphorylated aggregates. This pathology creates a dual-insult mechanism central to ALS models:
-
Gain of toxic function: These cytoplasmic aggregates disrupt cellular processes, impairing proteostasis and axonal transport.
-
Loss of nuclear function: A deficit in nuclear TDP-43 leads to cryptic exon inclusion and splicing failures in critical genes like STMN2 and UNC13A.
This pattern, seen in most ALS cases except rare SOD1- and FUS-driven types, makes TDP-43 a prime disease-modifying target, often described as the "holy grail" in ALS research.
2. Why Traditional Therapeutic Approaches Have Failed
Despite its significance, TDP-43 has been considered “undruggable.” The failure of traditional therapies can be attributed to three primary barriers:
-
Intracellular localization: TDP-43 aggregates are within neurons, making them inaccessible to circulating antibodies.
-
CNS compartmentalization: The blood-brain barrier limits CNS exposure for large biologics.
-
Essential physiological function: Broad suppression of TDP-43 is detrimental, necessitating selectivity for pathological species.
Traditional small molecules lack specificity, while conventional monoclonal antibodies cannot sustain intracellular CNS engagement. These constraints led to the innovation of vectorized antibody platforms that redefine brain delivery of biologics.
3. The Vectorized Antibody Concept
VTx-002 shifts from episodic drug administration to genetically encoded, sustained biologic therapy.
3.1 Platform Architecture
-
Vector: Adeno-associated virus serotype AAV5.2
-
Cargo: Gene encoding a selective anti-TDP-43 antibody fragment (an “intrabody”)
-
Administration: Single intracisterna magna (ICM) injection
After administration, CNS cells start continuous endogenous production of the therapeutic antibody, ensuring prolonged intracellular target engagement.
3.2 Intracellular Selectivity
The intrabody is designed to:
-
Bind misfolded, cytoplasmic TDP-43 aggregates
-
Spare native nuclear TDP-43, thus preserving its essential functions
This selectivity is vital for minimizing toxicity from indiscriminate TDP-43 depletion, distinguishing VTx-002 from previous strategies.
4. Mechanism of Action
Once expressed in CNS cells, VTx-002 works through three related processes:
-
Neutralization of cytoplasmic aggregates, reducing stress and disruption
-
Facilitation of aggregate clearance via endogenous degradation pathways
-
Restoration of nuclear TDP-43 function, normalizing RNA splicing
Preclinical studies showed reversal of cryptic exon inclusion in STMN2 and UNC13A, critical for motor neuron survival. These molecular changes provide a mechanistic readout of target engagement beyond nonspecific markers.
5. Preclinical Validation
VectorY conducted a thorough preclinical program for VTx-002, including:
-
Rodent and non-human primate models
-
CNS biodistribution mapping post-ICM delivery
-
Long-term expression and safety monitoring
-
Transcriptomic normalization of TDP-43-dependent splicing defects
This data supported the IND clearance in late 2025 by demonstrating biological plausibility, delivery feasibility, and an acceptable safety profile.
6. Clinical Development: PIONEER-ALS
6.1 Trial Design
-
Study name: PIONEER-ALS
-
Phase: 1/2
-
Design: Open-label, dose-escalation
-
Population: Approximately 12 adults with ALS
6.2 Primary Objective
-
Safety and tolerability of a single intracisterna magna dose
6.3 Exploratory Endpoints
-
Neurofilament light chain (NfL) trajectories
-
Novel TDP-43 pathway biomarkers
-
Clinical measures, including ALSFRS-R, slow vital capacity, hand-held dynamometry, and survival
The study is not powered for clinical efficacy, focusing instead on biological validation.
7. Regulatory Acceleration
In January 2026, the FDA awarded Fast Track designation to VTx-002, acknowledging:
-
ALS as a fatal disease with significant unmet medical need
-
VTx-002’s novel mechanism targeting the disease's biology
Fast Track status facilitates frequent regulatory interaction, potential rolling review, and eligibility for accelerated pathways if promising clinical or biomarker signals arise.
8. Implications Beyond ALS: Cognitive TDP-43 Disorders
While VTx-002 is currently focused on ALS, its mechanism could apply to frontotemporal lobar degeneration (FTLD) subtypes linked to TDP-43 pathology.
Among these, semantic-variant primary progressive aphasia (svPPA / semantic dementia) shows the strongest correlation with TDP-43 type C pathology, making it a likely target for cognitive applications.
From a translational viewpoint, svPPA offers benefits such as:
-
Pathology-enriched human model
-
Predominantly cortical involvement, distinct from motor neuron disease
-
Slower progression, suitable for biomarker studies
Any FTD expansion would likely follow proven human safety and target engagement in ALS, starting with exploratory trials.
9. Risks and Open Questions
Despite its potential, VTx-002 poses challenges:
-
Irreversibility of AAV delivery (“one-way door” problem)
-
Potential long-term immune responses to the vector or transgene
-
Translational uncertainty between motor and cognitive phenotypes
These risks highlight the importance of cautious, biomarker-driven development and emphasize early mechanistic validation over premature efficacy claims.
10. Conclusion
VTx-002 is a mechanistically rigorous approach to targeting TDP-43 proteinopathy, the key molecular driver of ALS. By combining gene therapy delivery, intracellular antibody engineering, and pathology-aligned biomarkers, VectorY has positioned VTx-002 as a potential disease-modifying intervention, beyond merely symptomatic relief.
If human safety and biological engagement are confirmed, the implications could extend to a wider range of TDP-43-mediated neurodegenerative disorders, potentially shifting the paradigm in treating intracellular proteinopathies in clinical medicine.